Research
Our research focuses on nanomorphology as an important level of biological membrane organization, exploring how membrane structure itself serves as a direct source of biological function. We investigate the fascinating world of membrane self-organization, particularly the formation of complex periodic arrangements with emphasis on cubic membranes that resemble triply periodic minimal surfaces known in differential geometry and topology.
Through an interdisciplinary approach that bridges biology, biophysics, and topology, we seek to answer important questions: How do biological membranes self-organize into complex three-dimensional periodic structures? What are the structural pathways of membrane transformations from one geometric configuration to another? What molecular mechanisms govern these transformations?
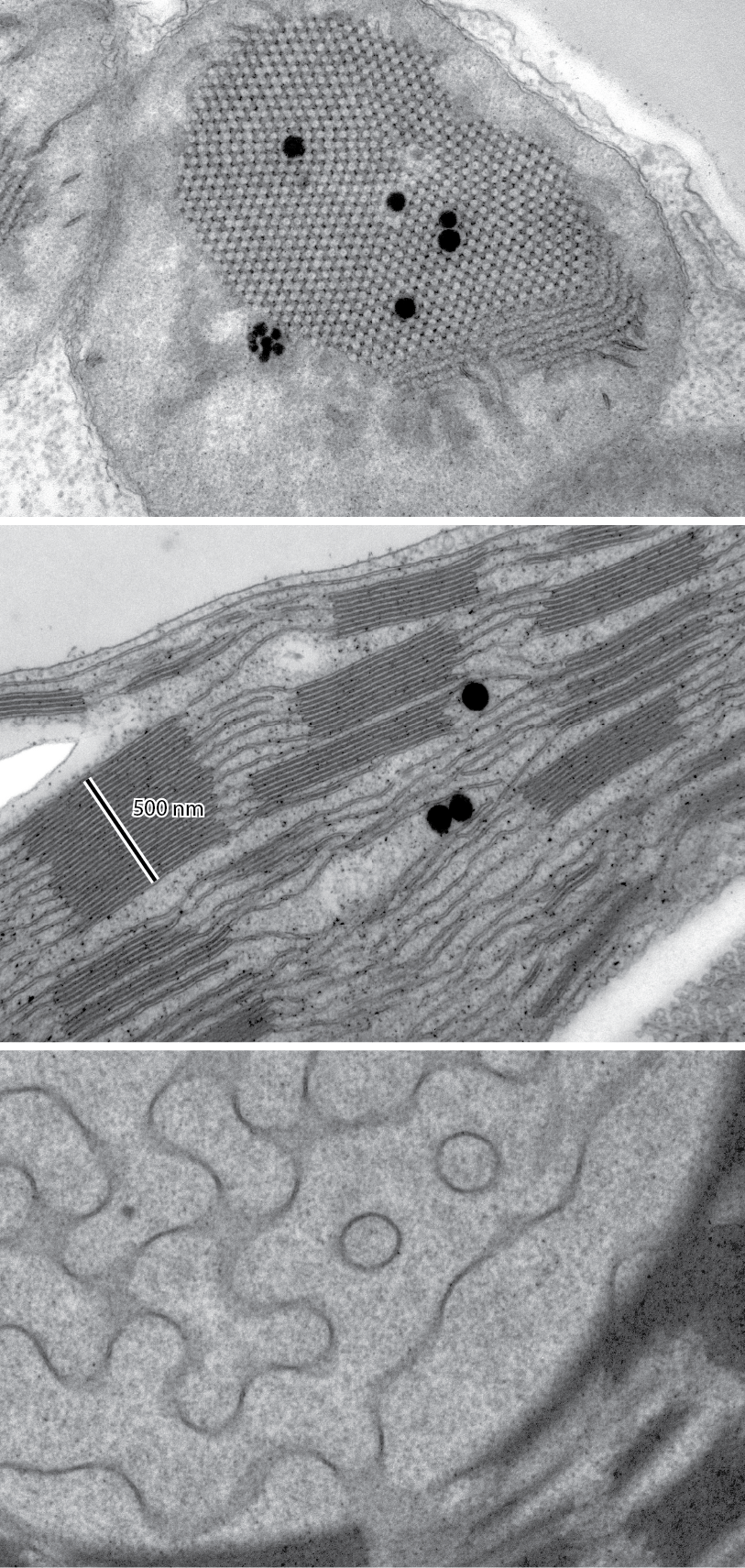
Our group investigates the structural plasticity of plant plastid internal membrane networks, using them as model systems to explore principles of membrane organization that have broader biological significance. We study both early membrane formation processes and transformations in mature chloroplasts, revealing connections between composition, structure, and function of these complex spatial membrane assemblies.
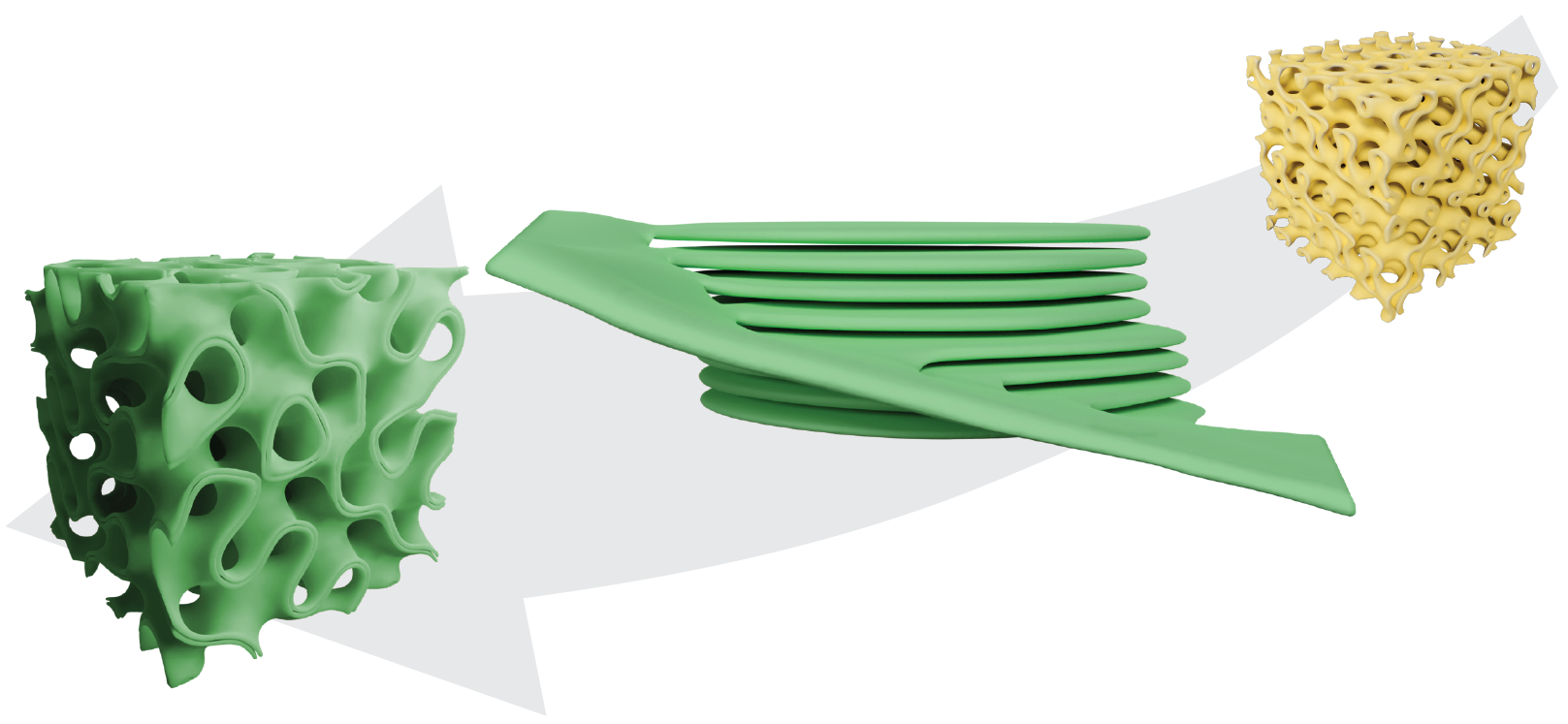
Using advanced 3D microscopy techniques (TEM, electron tomography, confocal microscopy) combined with computational modeling, biochemical and biophysical methods, we have deciphered key mechanisms of membrane transformation during chloroplast biogenesis. Our research revealed the three-dimensional ultrastructural transformation from the diamond-type cubic prolamellar body (PLB) to the lamellar thylakoid network. Our contributions include identifying the critical role of major lipids in forming periodic plastid membrane arrangements, with MGDG determining the helical organization of grana necessary for maintaining proper balance between cyclic and linear electron transport. We’ve demonstrated the structural role of carotenoids in forming and stabilizing both cubic and lamellar membrane systems, and identified the presence and role of CURT1 proteins in creating cubic PLB structure and regulating its transformation rate during chloroplast biogenesis.
To advance our research, we’ve developed specialized open-source software tools for reliable analysis of TEM images of plant membranes. Our SPIRE software enables identification of bicontinuous membrane structures from TEM sections through interactive matching against mathematical “nodal surface” models, representing a significant methodological contribution to the field.
We’ve also developed GRANA (Graphical Recognition and Analysis of Nanostructural Assemblies), an AI-enhanced tool that recognizes grana structures on electron micrographs and generates structural parameters significantly faster than manual approaches. This software facilitates large-scale analysis of grana nanomorphological features across diverse plant species.
The findings from our research not only contribute to understanding the fundamentals of biological membrane self-organization but also open new possibilities for designing biocompatible synthetic systems with potential applications in food processing, drug delivery systems, and other biomimetic materials. By drawing inspiration from nature’s sophisticated membrane architectures, we are exploring how these principles can be applied to create structurally complex biomimetic systems for technological applications.
Our work would not be possible without broad interdisciplinary collaboration. The expertise and contributions of researchers from different disciplines—including biophysicists, mathematicians, and computational scientists—have been valuable to our research. These collaborative efforts, involving researchers from leading centers in Europe and worldwide, reflect the interdisciplinary nature of our approach that combines cutting-edge biological questions with advanced methodological solutions.